
Green Transportation: Electric Vehicles in Latin America
Electric vehicles are a critical part of a clean transport agenda, but strong policy incentives are needed to promote widespread EV adoption in Latin America.
A Daily Publication of The Dialogue
Although several countries in Latin America and the Caribbean, including Mexico, Costa Rica and Chile, have begun inoculation programs against the novel coronavirus, the vaccine will not be widely available in most of the region until the second half of this year, next year or even 2023, according to the Economist Intelligence Unit. How do vaccination plans in Latin America and the Caribbean compare to those of the United States and other parts of the world? What sorts of problems have governments and health systems that have started the rollouts encountered so far? Which countries are best prepared for extensive inoculation, and what do you foresee as a realistic schedule for widespread and effective vaccination in the region?
María Luisa Ávila, chief of the infectiology service at the National Children’s Hospital in San José and former Costa Rican health minister: “Vaccination plans in Latin America and the Caribbean are not comparable to plans elsewhere because the realities are very different. Latin America and the Caribbean have an advantage of a long tradition of vaccination, success in the control of preventable diseases through vaccines and weak anti-vaccine movements. Of course, in economic terms, the higher-income countries have more options to acquire vaccines in addition to being more capable of ‘monopolizing’ vaccine production. However, the general idea is that access to vaccines is fair and equitable. Perhaps the biggest problem is vaccination logistics—having cold rooms to store the Pfizer vaccine, transportation to vaccination centers and the ability of local authorities to administer vaccines in the shortest time possible. There are also problems with the definition of risk groups, and the obvious desire of several groups to be considered a priority. I would also add questioning among the population of the speed with which the vaccines have been produced and criticisms against some vaccines, such as in Argentina with the Gamaleya-Sputnik V vaccine of Russian origin. The platforms that have led to vaccines have been around for a long time, a lot of economic and human resources have been invested in the development of vaccines and regulatory agencies, without compromising safety, and priority has been given to data analysis needed for the vaccines’ authorization. All countries have their weaknesses and strengths; perhaps smaller countries with fewer geopolitical problems will be the most successful, as will countries that have opted for having national health systems focused on public health, which are capable of properly conducting the process. Latin America and the Caribbean can be very successful given that the region has the practical and logistical knowledge of solid national vaccination programs. A realistic scheme includes starting with high-risk and rapid response personnel, then older adults, people at risk and then the general population, aiming for coverage of between 60-80 percent of the target population, with both doses of the current vaccines. Eventually, the possibility of single-dose regimens, for which studies are already being carried out, is encouraging.”
Katherine E. Bliss, senior fellow at the CSIS Global Health Policy Center: “With more than 15 million confirmed cases of Covid-19, Latin America and the Caribbean have been hit hard by the pandemic, making the successful distribution of vaccines to prevent infection critically important to the region’s recovery. A strong record of achievements in immunizations, including the elimination of polio and rubella, as well as vaccine manufacturing capacity in several countries, give the region a solid base of expertise upon which to build. But even before the pandemic, there were signs of strain within immunization programs, with stagnating coverage and sporadic measles outbreaks pointing to challenges related to health equity and access to basic services. Most countries in the region have joined COVAX, the international facility negotiating the purchase and distribution of vaccine doses through the Access to Covid-19 Tools (ACT) Accelerator. But COVAX is likely to provide enough vaccines for just 20 percent of participating countries’ populations over the next several months. To secure additional doses, several countries have hosted clinical trials of promising vaccine candidates, while others have pursued bilateral deals with multiple manufacturers, including Pfizer/BioNTech, Janssen, Sinovac and Gamaleya Research Institute, which produces Sputnik V, the Russian vaccine. Cuba is reportedly developing its own vaccine, while Mexico, Argentina and Brazil have signed agreements with Oxford/AstraZeneca to manufacture its vaccine locally. While local production of vaccines and procurement through mechanisms such as COVAX may help lower prices, countries can also prepare for a smooth distribution by identifying priority groups, training health workers to deliver the vaccines and building confidence in Covid-19 immunization programs.”
Arachu Castro, Samuel Z. Stone Chair of Public Health in Latin America at Tulane University’s School of Public Health and Tropical Medicine, and Paulo M. Buss, president of the American Alliance of Global Health: “Effectively controlling the Covid-19 pandemic will require the deployment of a systematic vaccination strategy to ensure that a large portion of every country’s population receives the vaccine. Incoherent with this basic epidemiological principle, some countries are procuring more vaccines than needed to cover their own populations, while undermining low- and middle-income countries’ ability to access, or even produce, enough vaccines to guarantee herd immunity. Unless governments and the pharmaceutical industry take urgent action to ensure that enough Covid-19 vaccine doses are produced, the majority of Latin American countries will only be able to vaccinate one in five people against Covid-19 by the end of 2021. Chile and Mexico have purchased enough doses of the most promising vaccines to cover more than 100 percent of their population, and three other countries have a potential coverage greater than half of their population: Peru (74 percent), Brazil (63 percent) and Argentina (53 percent)—according to Duke’s Launch and Scale Speedometer. Without an equitable distribution of Covid-19 vaccines throughout Latin America, the regional economy will not recover, causing greater human suffering and premature mortality. Therefore, to protect people’s lives, an effective and safe Covid-19 immunization strategy should be considered a global public good, and vaccines should be produced at larger volumes, distributed equitably between countries and provided at no cost to the user. This can be achieved by waiving intellectual property rights to vaccines, tests and treatments related to Covid-19, openly sharing the vaccines’ technology and intellectual property through the World Health Organization (WHO), fully funding COVAX, stopping bilateral deals, supporting WHO efforts and investing in the strengthening of national health systems. Governments should abide by their human rights obligations and promote, as opposed to hinder, worldwide access to the vaccine.”
Cuauhtémoc Ruiz Matus, chief of the Pan American Health Organization’s Immunization Program: “PAHO is working with member states to ensure their immunization programs are well prepared to roll out Covid-19 vaccines as they receive them. This includes support with vaccine planning, logistics and cold-chain management, strengthening surveillance of adverse effects following immunization, as well as information systems, training of health care workers, generating vaccine demand and communication. COVAX is part of the ACT accelerator, for which the GAVI Alliance is serving as an administrator. All Latin American and Caribbean countries that participate in COVAX will do so through the PAHO Revolving Fund, and there won’t be independent purchases with those specific producers outside COVAX. As part of the COVAX facility, PAHO timelines for distribution are tied to that of the facility. Some countries have made direct agreements with different manufacturers for the procurement of vaccines. Each country has its own national regulatory authorities, analogous to the FDA in the United States, which approves the vaccines they will use. PAHO can support these countries in the adoption of appropriate regulatory frameworks to ensure that they can implement these procedures using international standards. For PAHO to procure a vaccine on behalf of the COVAX facility, the vaccine must be prequalified by WHO or listed under the WHO Emergency Use Listing (EUL) procedure. So far, WHO has approved one vaccine under that scheme, the Comirnaty Covid-19 mRNA vaccine for emergency use, making the Pfizer/BioNTech vaccine the first to receive emergency validation from WHO since the coronavirus outbreak began. The EUL opens the door for countries to expedite their own regulatory approval processes to import and administer the vaccine. It also enables UNICEF and PAHO to procure the vaccine for distribution to countries in need. PAHO is also monitoring where countries are in terms of readiness for vaccines, using the Covid-19 Vaccine Introduction Readiness Assessment Tool (VIRAT), a tool for national authorities with a roadmap to monitor progress in the introduction of the vaccines. It covers regulations, COVAX agreements, national coordination bodies, the designation of primary target groups for the vaccines, cold-chain capacity and other factors.”
Felicia M. Knaul, director, and Michael Touchton, researcher, at the University of Miami’s Institute for Advanced Study of the Americas: “Europe, Canada and the United States are challenged by the daunting task of rapidly reaching the majority of people with vaccines that require strong supply chains as they face reticence in the population. In Latin America and the Caribbean (LAC), rollout is significantly more difficult. LAC health systems, while stronger than many in other developing regions, tend to be fragmented and chronically underfunded and have been under extreme duress battling the pandemic for almost a year. Even pre-pandemic, health spending was a fraction of what Canada invests: less than a third in Chile, 10 percent in Mexico and 5 percent in Guatemala and Bolivia. The cost of the vaccines and of their distribution poses a heavy burden. Price negotiation is key. LAC countries may resort to cheaper vaccines, such as Russia’s Sputnik (in Argentina) or China’s vaccine, which is undergoing testing in Brazil. These vaccines may be less effective than more expensive options. Pandemic politization further hinders rollout. In Brazil, President Bolsonaro declared syringes too expensive, calling for a halt on government purchases until prices fall. In Mexico, infection and death rates are persistently high, and the health system was destabilized in early 2020 by the closure of the Seguro Popular. In both countries, the toughest aspects of pandemic management were relegated to weaker state-level health systems. Problems plague low- and middle-income countries worldwide, but Latin America has been a Covid-19 hotspot for months. While the huge need for vaccines is met, LAC must apply an evidence-based public health approach relying on mask use, testing and outbreak management and adopting new treatment protocols.”
 The Latin America Advisor features Q&A from leaders in politics, economics, and finance every business day. It is available to members of the Dialogue’s Corporate Program and others by subscription.
The Latin America Advisor features Q&A from leaders in politics, economics, and finance every business day. It is available to members of the Dialogue’s Corporate Program and others by subscription.
Electric vehicles are a critical part of a clean transport agenda, but strong policy incentives are needed to promote widespread EV adoption in Latin America.
In Brazil, the possibility of pregnant women with Zika having access to abortion has not entered the public debate.
Lawmakers join the Dialogue and the O’Neill Institute for National and Global Health in open discussion on the Zika virus as a global health crisis.
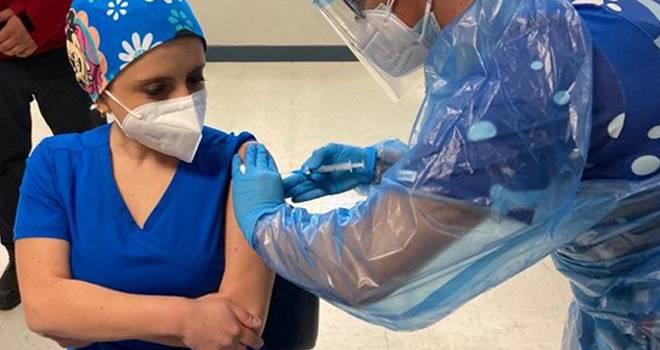 Some countries in Latin America have started vaccinations against Covid-19. A health care worker in Chile is pictured getting the inoculation last month. // Photo: Chilean Government.
Some countries in Latin America have started vaccinations against Covid-19. A health care worker in Chile is pictured getting the inoculation last month. // Photo: Chilean Government.

